Dry Cell Car Batteries The Reliable Automotive Power
Contents

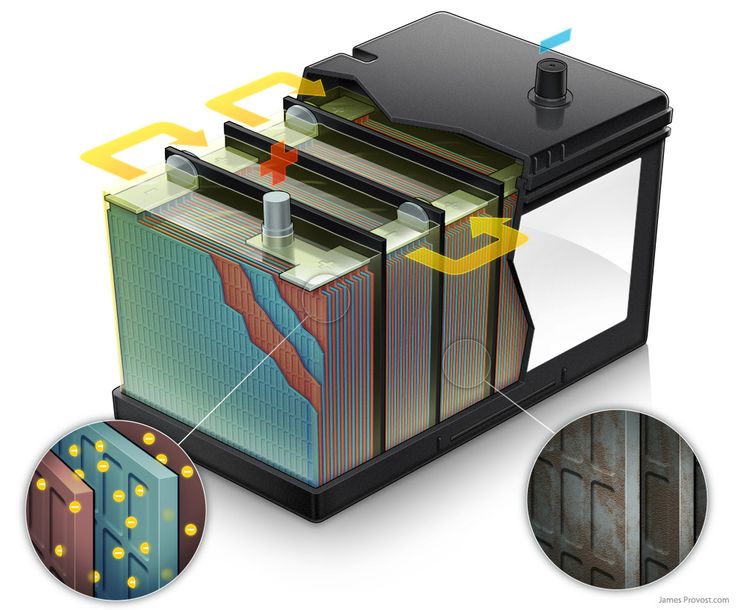
Dry cell car battery
A device is made of further electrochemical cells that turn stored chemical energy into electrical energy. Most motor vehicles depend on lead-acid batteries or lithium-ion batteries for their power needs. If you are interested in investigating the concept of using dry-cell batteries in cars, here are some outline ideas to consider.
Introduction
A dry cell contains a metal in which low-moisture electrons paste to cover the graphite rod or metal electrode. The lead acid battery constitutes the reversible battery technology. It can be established in a wide range of applications, including small-scale power storage such as ups ( uninterruptible power supply) systems.
A dry cell battery is a device made of more electrochemical cells that convert stored chemical energy into electrical energy. It consists of an electrolyte that is contained within a paste or other wet medium. Standard dry cell batteries involve a zinc anode and a carbon within a central rod.
Challenges and Restriction
- Dry cell car batteries are suitable because they are easy to store and don’t require any preservation. Moreover, dry batteries can leak if they are damaged, which causes rust and other problems. The restriction of dry cell car batteries is if the chemicals inside the battery are defined to an extreme amount of heat, dry cell batteries can break and blast.
Potential Applications
- Before, in a dry cell, the electrolyte, in a dry cell, the electrolyte, similarly ammonium chloride, is dry. The voltage composed by a fresh dry cell is 1.5V but reduced during use.
- Such dry cells are most common and used in radio and toys.
- Liquid electrolytes, like sulfuric acid, pose potential hazards due to their corrosive nature. Sulfuric acid is a particularly dangerous substance known for its corrosive properties.
Research and development
German scientist Carl Gassner developed the dry cell after the development of wet zinc-carbon batteries by Georges Leclanch’e in 1866.
In 1887, a Japanese inventor named Sakizo Yai developed a specific type of dry cell. The following year, in 1888, a German inventor named Carl Gassner created a groundbreaking battery design that eliminated the risk of the solution spilling. Despite containing a liquid, Gassner’s invention was termed a “dry cell” or “dry battery” because it effectively prevented any leakage or spillage, marking a significant advancement in battery technology.
Principle of dry cell batteries
The simple principle of dry cell batteries is electrochemical cells. That converts electrochemical cells into electrical energy. They were developed by George Leclanche in 1866., and are also called leclanche cells.
Dry Cell Car Batteries The Reliable Automotive Power

Safety consideration of dry cell car batteries
- Never overloaded with acid.
- Never allow children access to a battery.
- Never allow battery air to become blocked.
- Always put on eye protection.
- Always store upright.
- Always charge in a well-ventilated area.
- Always wear protective clothing.
- Construction and working of dry cell car batteries
- The modern valve-regulated, lead-acid (VRLA) battery is sealed and does not require any topping up of water.
- Always wear protective clothing.
Construction and working of dry cell car batteries

The modern valve-regulated, lead-acid (VRLA) battery is sealed and does not require any topping up of water.
A dry cell is a convenient form of a leclanche cell. It contains a zinc vessel that acts as a negative electrode or anode. The ship includes a humid paste of sawdust soaked with a solution of ammonium chloride and acts as an electrolyte.
Dry cell batteries are made of one or more electrochemical energy into electric energy. A dry cell battery consists of a metal container in which low-moist electrolyte paste covers the graphite rod or a metal rod.
The metal casing of the battery is typically made of zinc, which serves as the negative electrode, also known as the anode. Meanwhile, a carbon rod functions as the positive electrode, referred to as the cathode). It consists of a brass cap, air light seal, electrolyte, carbon rod, which is a positive electrode, and zinc container, which is a negative electrode.
170 CCA; Better warranty: Limited 2-year full replacement warranty, not pro rata; Longer service life:

Pros of dry cell car battery
- They are very low-preservation
- Dry cell car batteries are small in size
- . Dry cell car batteries are lightweight; that way, dry cell car batteries can be carried from place to place, and there is no risk of leakage of chemicals in a dry cell.
- Dry cell batteries are less expensive.
- The electrolyte used in dry cells is relatively not so harmful to the environment.
Cons of dry cell batteries
- have several damaging environmental significance when they are ready or in a landfill.
- Dry cell car batteries can break and blast if the chemicals inside the battery are wreaked to extreme heat.
- If the chemicals in the storm are bare to an excessive amount of heat, the dry cell batteries can crack and blast.
- The chemicals that are used in dry-cell car batteries damage the skin.
- Dry cell batteries have several detrimental environmental implications when they are disposed of in landfills.
Conclusion
A device is made of further electrochemical cells that turn stored chemical energy into electrical energy. Most motor vehicles depend on lead-acid batteries or lithium-ion batteries for their power needs. If you are interested in investigating the concept of using dry-cell batteries in cars, here are some outline ideas to consider.
FQA
Are dry cell batteries flammable?
Dry cell batteries are highly explosive when heated. There is a need to train the commonality about their volatile nature to keep batteries away from children.
What are the applications of dry cell batteries?
A Dry cell battery is a movable electric battery that is used mainly in homes for Flashlights, radios, remote controllers, and many other devices. It transforms chemical energy like other batteries.
Who discovers dry battery?
Carl Gasser discovered the dry batteries. More improvements came in the 1880s when German scientist ( Carl Gasser) invented the first dry-cell battery.
How many types of dry cell batteries?
The types of dry cell batteries are:
1: Zinc carbon batteries
2: Alkaline cell batteries.
3: Mercury batteries.